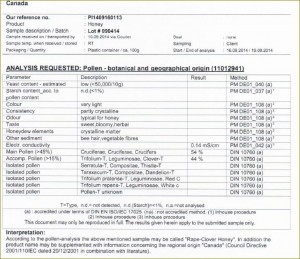
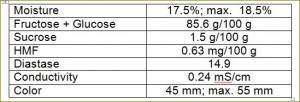
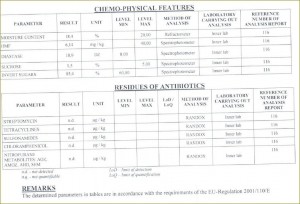
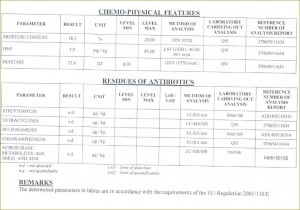
Definitions of honey
According to Merriam Webster dictionary, honey is a sweet viscid material elaborated out of the nectar of flowers in the honey sac of various bees. Wikipedia defines honey as a sweet food made by bees using nectar from flowers.
Codex Alimentarius Standard for Honey (Codex Stan 12-1981)
 “Honey is the natural sweet substance produced by honey bees from the nectar of plants or from secretion of living parts of plants or excretions of plant sucking insects on the living parts of plants, which the bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store and leave in the honey comb to ripen and mature.”
“Honey is the natural sweet substance produced by honey bees from the nectar of plants or from secretion of living parts of plants or excretions of plant sucking insects on the living parts of plants, which the bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store and leave in the honey comb to ripen and mature.”
US FDA definition and description of honey
 (see FDA Standards for honey)
(see FDA Standards for honey)
Basically, the US Food and Drug Administration (FDA) was actively working closely with the World Health Organization and Food and Agriculture Organization of the United Nations on the development of internationally accepted standards for honey. Therefore the below quotation from an FDA document describing the FDA view of standards for honey as of March 2006 is in essence the acceptance of the above Codex Alimentarius standards:
“The US Food and Drug Administration (FDA) and other U.S. interests participate in the development of international standards by the Codex Alimentarius Commission, an international organization formed in 1962 to facilitate world trade. In 2001 the revised Codex Standard for Honey was adopted by the 24th Session of the Codex Alimentarius Commission. The United States participated fully in the proceedings. While honey is produces in the U.S., traded internationally and consumed both as a packaged food and as a food ingredient, there currently is no standard of identity for honey in the U.S. law.”
Paragraph 2.1 of Exhibit C of the document reads:
“2,1 DEFINITION
Honey is the natural sweet substance produced by honey bees from the nectar of plants or from secretion of living parts of plants or excretions of plant sucking insects on the living parts of plants, which the bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store and leave in the honey comb to ripen and mature.”
Also, here is the FDA description of honey:
“2.2 DESCRIPTION
Honey consists essentially of different sugars, predominately fructose and glucose as well as other substances such as organic acids, enzymes and solid particles derived from honey collection. The color of honey varies from colorless to dark brown. The consistency can be fluid, viscous or partly to entirely crystallized. The flavor and aroma vary, but derived from the plant origin.”
Classification of honey
Types of Honey by USDA (see United States Standards for Grades of Extracted Honey):
- Liquid honey is honey that is free of visible crystals.
- Crystallized honey is honey that is solidly granulated or crystallized, irrespective of whether candied, fondant, creamed, or spread types of crystallized honey.
- Partially crystallized honey is honey that is a mixture of liquid honey and crystallized honey.
- Filtered honey is honey of any type that has been filtered to the extent that all or most of the fine particles, pollen grains, air bubbles, or other materials normally found in suspension, have been removed.
- Strained honey is honey of any type that has been strained to the extent that most of the particles, including comb, propolis, or other defects normally found in honey have been removed. Grains of pollen, small air bubbles, and very fine particles would not normally be removed.
It is widely accepted that raw honey is honey which was not filtered or heated above normal ambient temperature.
Honey may be classified as
- Monofloral (from a single botanical source). Monofloral honey may be classified by the specific blossom-type from which the nectar is extracted (e.g., blueberry honey made from nectar obtained from blueberry blossoms). Often, specialty monofloral honeys sell at a premium price.
- Polyfloral (from multiple botanical sources, with no single predominant floral source). Polyfloral honey, having been derived from a mixture of many floral nectars, is generally classified by the time of the year it is harvested rather than by floral sources (e.g., spring honey or autumn honey); however, polyfloral honey may also be classified by the area from which the nectar was sourced such as “desert honey” or “mountain honey.”
While many varieties of honeys exist on the market, most honey is blended to achieve a desired color and flavor as well as to produce uniform product throughout a given market, most natural honey produced in the United States is marketed in liquid form, which is honey that is extracted from the comb by centrifugal force, gravity, or straining.
Natural honey is also marketed as
- Cream honey (also called “creamed,” or “whipped”), which consists of pure honey in which glucose crystallization has been encouraged;
- Comb honey, which is honey marketed in the beeswax comb, both of which are edible;
- Cut comb honey, which is liquid honey that has been packaged with chunks of honey comb; and
- Dry honey (also known as “dried” or “powdered” honey), which is made by removing the water found in liquid honey by drum- or spray-drying.
Honey embodies mild antiseptic properties and has been used in the treatment of minor skin injuries such as burns and lacerations.
Obviously, honey is a valuable natural food product. Honey was recently listed as one of the 11 healthiest foods in the world: “It is rich in antioxidants and is often used as an antiseptic treatment on wounds. As Rodale said, it also contains phytoestrogens, and studies on Greek honey have found that those phytoestrogens can blunt the growth of breast, prostate, and endometrial cancers. Honey also has a low glycemic index, so using it to sweeten tea or coffee won’t lead to energy-busting blood sugar drops later in the day.”
However, since honey was turned to a commodity long time ago, it has been unintentionally and intentionally denatured, altered and adulterated by addition of water, sugar or high fructose corn syrup or by removal of pollen often for profit gain (See: Honey Bee Hoarding of High Fructose Corn Syrup and Cane Sugar Syrup, 1980). As the result, by 2011 two thirds of the honey found in the US stores was reported denatured. Accordingly, to distinguish between natural and denatured honeys the original simple definition of honey should have been complemented by additional characteristics such as water content, sugar (sucrose) content, diastase activity, and pollen presence to name a few. FDA: Honey with any added sweeteners isn’t honey. The agency said enforcement action is possible against U.S. food businesses or importers if companies try to cut those sweeteners into real honey and do not label the product correctly. If those sweeteners are added, the label should read “blend of sugar and honey” or “blend of honey and corn syrup. The FDA regularly detains honey imports and tests them after finding drug residues and unlabeled added sweeteners.
Quality standards for honey
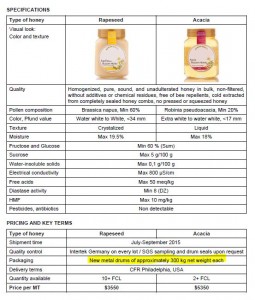
Quality of honey is characterized by three sets of parameters:
- Organoleptic properties reflect what can be sensed and described based on sight, taste, smell, and mouthfeel. Those are: color, aroma, taste and texture.
- Natural chemical composition of honey is formed by water, glucose, fructose, reducing sugars, sucrose, protein, enzymes and vitamins content.
- Level of chemical contamination caused by residual antibiotics, pesticides and heavy metals. Added sugars like HFCS or artificial sweeteners can also be measured.
Color of honey
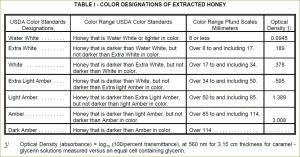
Grading according to color has long been an important factor in marketing of honey. Currently USDA uses spectrophotometric approach to systematically grade honey into seven color designations (see United States Standards for Grades of Extracted Honey):
- Water White (means colorless)
- Extra White (darker)
- White
- Extra Light Amber (ELA)
- Light Amber (LA)
- Amber
- Dark Amber
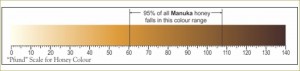
Pfund Scale for Honey Color
Earlier a semi-quantitative scale was devised to describe the color of honey. Measurements were originally made with the Pfund color grader, which consisted of a wedge of amber-colored glass next to a wedge-shaped cell which is filled with honey. The instrument is read visually; the reading is the distance the wedge must be moved to make a match, and is expressed in millimeters.

Pfund Grader
While the Pfund color grader is not the officially approved device for determining color designation for the USDA grade standards of honey, it is still popular in the honey industry. Instead, according to the USDA, transmittance is measured at a wavelength of 560 nanometers, through 3.15 centimeters of a freshly-prepared caramel-glycerin solution that matches the color of the honey, as compared with the transmittance of pure glycerin. The optical density (absorbance), as given in the last column in the table below, is log to the base 10 of 100 divided by the percent transmittance (see Definition of Honey Color Grades).
 According to Bruce Boynton, CEO of the National Honey Board, nearly 50% of U.S. consumers want honey that’s clear and golden, and that about 25% prefer raw, unfiltered honey. The floral source grants honey its distinctive flavor (e.g., star thistle, orange blossom, sage, and clover) and its distinctive color (e.g., “water white” or “dark amber”). Generally, lighter colored honeys possess a more mild flavor (e.g., clover honey), while darker colored honeys possess a stronger flavor (e.g., buckwheat honey). Honey is valued on the basis of floral source and color, and in most countries, the light-colored and milder tasting honeys are considered to be more valuable.
According to Bruce Boynton, CEO of the National Honey Board, nearly 50% of U.S. consumers want honey that’s clear and golden, and that about 25% prefer raw, unfiltered honey. The floral source grants honey its distinctive flavor (e.g., star thistle, orange blossom, sage, and clover) and its distinctive color (e.g., “water white” or “dark amber”). Generally, lighter colored honeys possess a more mild flavor (e.g., clover honey), while darker colored honeys possess a stronger flavor (e.g., buckwheat honey). Honey is valued on the basis of floral source and color, and in most countries, the light-colored and milder tasting honeys are considered to be more valuable.
Perhaps, there is erroneous belief among many consumers that “light and clear” honey is somehow cleaner and healthier than dark, translucent or opaque honey. The truth is right the opposite. The darker a honey is the more peroxide fighting antioxidants it contains which means more healing power the honey may produce. Again, the darker a honey is the more it is distant from high fructose corn syrup in terms of quality and the more valuable it is. Want analogy? Think of white (artificial) vs dark chocolate!
Natural chemical composition of honey
(See: FDA Standards for honey. Exhibit B)
Honey is a sweet viscous fluid derived from the nectar of flowers and produced in the honey sac of bees. Honey is an invert sugar, composed mainly of simple sugars (called monosaccharaides, such as fructose and glucose) and water. Fructose and glucose comprise approximately 70% of the content of honey while water comprises about 17%. The remaining components of honey are maltose, sucrose (commonly called “table sugar”), and other complex carbohydrates. Also, honey contains essential vitamins (e. g., vitamin B6, thiamin, niacin, riboflavin, and pantothenic acid), minerals (e.g., calcium, copper, iron, zinc, and magnesium), several different amino acids, and several antioxidant compounds (e.g., vitamin C and chrysin). Honey is classified by its individual characteristics (e.g., floral source, color, season, physical state, and means of preparation. There are over 300 unique varieties of honey that are produced in the United States. Honey differs in flavor and color depending upon the floral source from which the nectar is extracted by the honey bee.

Colors of honey
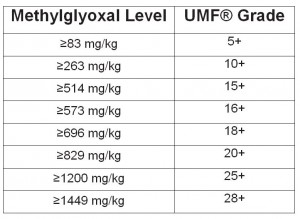
Biological activity of honey is characterized by diastase number. Diastase is a group of enzymes added by bees during honey production for the breakdown of starch into maltose. (see: Measurement of Honey Quality). Read more about diastase here.
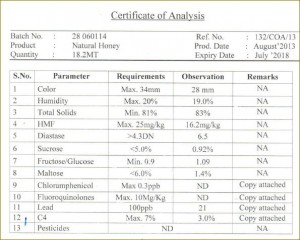
| Constituent Content |
Limits |
| Moisture (water) |
Not more than 20% (< 20%) |
| Sum of Fructose and Glucose |
Not less than 60 g/100 g (> 60%) |
| Sucrose |
Not more than 5g/100 g (< 5%) |
| Water insoluble solids |
Not more than 0.1 g/100 g (< 0.1%) |
| Free acidity |
Not more than 50 milliequivalents per 1000g; i.e., not more than (MW x 50)/1000 |
| Diastase activity |
Not less than 8 Schade units (see ) |
| Hydroxymethylfurfural (HMF) |
Not more than 40 mg/KgNot more than 80 mg/Kg for the honey from tropical ambient temperatures |
| Electrical conductivity |
Not more than 0.8 mS/cm-1 |
Please note that many national beekeeping organizations (e. g., Germany, Belgium, Austria, Italy, Switzerland) have moisture (water) maximum content required not to exceed 18 – 18.5%.
According to the Codex Alimentarius standards reviewed and accepted by the US FDA ”honey sold as such shall not have added to it any food ingredient, including food additives, nor shall any other additions be made other than honey. The honey shall not have begun to ferment or effervesce. No pollen or constituent particular to honey may be removed except where there is unavoidable in the removal of foreign inorganic or organic matter.”
Chemical contaminants in honey
Modern production of honey occurs in the conditions where monoculture practices and mass production of food have led to massive use of agricultural chemicals. As the result, today’s honey is produced in the environment polluted with fertilizers and pesticides. The chemical pollutants, in turn adversely affect biochemistry and health of bees bringing new problems such as Colony Collapse Disorder and other diseases. To cope with new and old challenges beekeepers are prompted to use animal drugs such as antiseptics and antibiotics.
PESTICIDES
Tolerances and exemptions for pesticide chemical residues in food as of Code of Federal Regulations Title 40, Part 180 (data for use in honey is only shown)
| Pesticide |
Parts per million, less than |
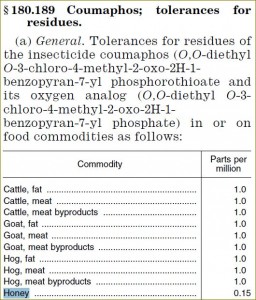
| §180.189 Coumaphos |
0.15 |
| §180.287 Amitraz |
0.2 |
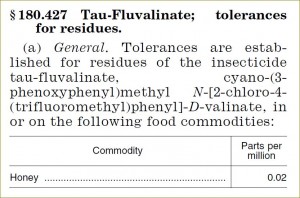
| §180.287 Tau-Fluvalinate |
0.02 |
|
|
| EXEMPTIONS from the requirement of a tolerance |
|
| §180.1011 Microbial insecticide Bacillus thuringiensis Berliner |
Exemption from the requirement of a tolerance is established for residues of Microbial insecticide Bacillus thuringiensis Berliner in or on honey and honeycomb when it applied to growing crops, or when it applied after harvest in accordance with good agricultural practices. |
| §180.1035 Pine oil |
Pine oil is exempted from the requirement of a tolerance for residues in the raw agricultural commodities honey and honeycomb, when present therein as a result of its use as a deodorant at no more than 12 percent in formulation with the bee repellent butanoic anhydride applied in an absorbent pad over the hive. |
| §180.1092 Menthol |
the pesticidal chemical in or on honey and honeycomb when used in accordance with good agricultural practice in over-wintering bee hives. |
| §180.1178 Formic acid |
when used to control tracheal mites and suppress varroa mites in bee colonies, and applied in accordance with label use directions. |
| §180.1271 Eucalyptus oil |
In or on honey, honeycomb, and honeycomb with honey when used at 2 g or less eucalyptus oil per hive, where the eucalyptus oil contains 80% or more eucalyptol. |

US EPA: What the Pesticide Residue Limits are on Food
EPA: Index to Pesticide Chemical Names and Tolerances
Codex Pesticides Residues in Food Online Database
Title 40 of the Code of Federal Regulations; Title 40: Protection of Environment (see CFR_2011_title 40 _vol 24_part180)
ANTIBIOTICS
In general, antibiotics should not be detected in honey. Oxytetracycline is commonly used in the USA and EU to control the outbreak of American foulbrood and European foulbrood. FDA has established maximum residue limits (MRL) called tolerances for residual animal antibiotics in food stated in the Code of Federal Regulations, Title 21, Part 556. However, there are no residue limits established for veterinary drugs particularly in honey that implies that they should be absent.
Similarly, the EU regulations for honey are expressed in the Council Directive 2001/110/EC. MRL values for residual antibiotics in foodstuffs are set in Regulation (EU) No 37/2010 which states that each antibiotic must have its MRL established before it can be used in a food producing animals. However, there are no MRL established for residual of veterinary drugs particularly in honey that means that EU does not allow any residual antibiotics in honey.
Codex Alimentarius provides MRL values for veterinary drug residues in food which can be found in an online database: Codex Veterinary Drug Residues in Food Online Database
The use of animal drugs in the United States and prohibited drugs
Code of Federal Regulations Title 21, Part 530 – Extralabel drug use in animals
Extralabel use means actual use or intended use of a drug in an animal in a manner that is not in accordance with the approved labeling. This CFR applies to the extralabel use in an animal of any approved new animal drug or approved new human drug by or on the lawful order of a licensed veterinarian within the context of a valid veterinary-client-patient relationship. The purpose of CFR is to establish conditions for extralabel use or intended extralabel use in animals by or on the lawful order of licensed veterinarians of Food and Drug Administration approved new animal drugs and approved new human drugs.
(a) The following drugs, families of drugs, and substances are prohibited for extralabel animal and human drug uses in food-producing animals.
(1) Chloramphenicol;
(2) Clenbuterol;
(3) Diethylstilbestrol (DES);
(4) Dimetridazole;
(5) Ipronidazole;
(6) Other nitroimidazoles;
(7) Furazolidone.
(8) Nitrofurazone.
(9) Sulfonamide drugs in lactating dairy cattle (except approved use of sulfadimethoxine, sulfabromomethazine, and sulfaethoxypyridazine);
(10) Fluoroquinolones; and
(11) Glycopeptides.
(12) Phenylbutazone in female dairy cattle 20 months of age or older.
(13) Cephalosporins (not including cephapirin) in cattle, swine, chickens, or turkeys:
(i) For disease prevention purposes;
(ii) At unapproved doses, frequencies, durations, or routes of administration; or
(iii) If the drug is not approved for that species and production class.
(b) The following drugs, families of drugs, and substances are prohibited for extralabel animal and human drug uses in nonfood-producing animals: [Reserved]
(c) [Reserved]
(d) The following drugs, or classes of drugs, that are approved for treating or preventing influenza A, are prohibited from extralabel use in chickens, turkeys, and ducks:
(1) Adamantanes.
(2) Neuraminidase inhibitors.
Unsafe drug residues can be a reason for detention of an import shipment of honey (FDA Import Alert 36-04). Antibiotics are used in the treatment of honey bee diseases. However, certain drug residues are a concern to human health. In the United States, the Food and Drug Administration (FDA) has not approved the use of chloramphenicol, nitrofurans and/or fluoroquinolones as drugs to treat honey bees.
Extralabel uses of chloramphenicol, nitrofuran and/or fluoroquinolone drugs are prohibited in food producing animals. Therefore, if they are used as drugs to treat honey bees, they are considered to be unsafe new animal drugs within the meaning of Section 512. If there are residues of these drugs in honey as a result of treatments of the bees, the honey is deemed to be adulterated under section 402(a)(2)(C)(ii) of the FFDCA.
Furthermore, chloramphenicol, nitrofurans and/or fluoroquinolones are considered to be food additives if the intended use of these substances results in or may reasonably be expected to result in their becoming a component of food. Chloramphenicol, nitrofurans and/or fluoroquinolones are not generally recognized as safe for use in food, nor is there a food additive regulation authorizing their use in food. Consequently, residues of these drugs in foods are unsafe food additives within the meaning of Section 409 of the FFDCA and would render the food adulterated under section 402(a)(2)(C)(i).
Code of Federal Regulations Title 21, Part 556 – Tolerances for residues of new animal drugs in food
Unfortunately, there are no tolerances established for the residual drugs in honey. However, tolerances established for the residues in different types of food (or tissues) may serve as guidance. For instance, for Tetracycline the tolerances fall into 2 – 12 ppm while for Streptomycin into 0.5 to 2.0 ppm ranges depending on the type of food.
§556.500 Oxytetracycline
(a) Acceptable daily intake (ADI). The ADI for total tetracycline residues (chlortetracycline, oxytetracycline, and tetracycline) is 25 micrograms per kilogram of body weight per day.
(b) Beef cattle, dairy cattle, calves, swine, sheep, chickens, turkeys, finfish, and lobster. Tolerances are established for the sum of residues of the tetracyclines including chlortetracycline, oxytetracycline, and tetracycline, in tissues and milk as follows:
(1) 2 parts per million (ppm) in muscle.
(2) 6 ppm in liver.
(3) 12 ppm in fat and kidney.
(4) 0.3 ppm in milk.

Tolerances for Oxytetracycline in foods
§556.610 Streptomycin
Tolerances are established for residues of streptomycin in uncooked, edible tissues of chickens, swine, and calves of 2.0 parts per million (ppm) in kidney and 0.5 ppm in other tissues.
§556.720 Tetracycline
(a) Acceptable daily intake (ADI). The ADI for total tetracycline residues (chlortetracycline, oxytetracycline, and tetracycline) is 25 micrograms per kilogram of body weight per day.
(b) Tolerances. Tolerances are established for the sum of tetracycline residues in tissues of calves, swine, sheep, chickens, and turkeys, of 2 parts per million (ppm) in muscle, 6 ppm in liver, and 12 ppm in fat and kidney.
It worth noting that although there are established tolerances for Enrofloxacin (§556.226 Enrofloxacin, 0.1 – 0.5 ppm) by its chemical structure it does belong to the group of Fluoroquinolones listed above as prohibited drugs (§530.41). Obviously, best decision in such situations would be not to use Enrofloxacin or other controversial drugs in beekeeping.
Bottom line. If you are a honey exporter who considers export to the USA and/or Canada, you have to make all possible efforts in order to keep residual antibiotics in your honey supply below Limits of Detection that are in ppb range (1/1000 of ppm).
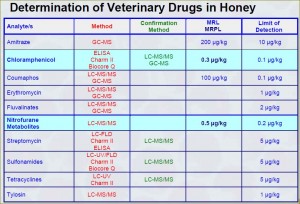
While it is understood that there should not be any residual antibiotics in honey, the authorities, taking into account the world’s realities, may accept residual antibiotics if:
- They are not prohibited
- They are in ppb range, not in ppm range.
Maximum Residue Limits for antibiotics in honey which may or may not be accepted by the USA and Canada:
TOXIC METALS
US FDA: Metal chemical contaminants in food
US EPA: Mercury
§556.60 Arsenic; Lead; Tin
EFSA on metals in food
For the heavy metals cadmium, lead and mercury, maximum levels in certain foods have been established by Commission Regulation (EC) No 1881/2006.
| Metal |
EFSA in foodstuffs, Less than mg/Kg |
EFSA, Less than |
FDA, Less than |
| Lead |
0.020 |
20 ppb |
50 ppb |
| Cadmium |
0.050 |
50 ppb |
|
| Mercury |
0.5 |
500 ppb (0.5 ppm) |
|
| Tin |
50 |
50000 ppb (50 ppm) |
200 ppm |
See US FDA: Juice HACCP Hazards and Controls Guidance First Edition
Lead is especially hazardous to young children. In 1993, FDA established an emergency action level of 80 ppb and above for lead in juice packed in lead soldered cans. (See the Federal Register notice of April 1, 1993 (58 FR 17233).) However, based upon a recent toxicological assessment for lead carried out by the Joint WHO/FAO Expert Committee on Food Additives, the Codex Alimentarius Commission, an international food standards organization that establishes safe levels for the protection of consumers, has recently established a maximum level of 50 ppb for lead in ready-to-drink fruit juices, including fruit nectars that are in international trade, to protect the public health. FDA concurs with this recent assessment that lead levels in juice above 50 ppb may constitute a health hazard, and FDA may in the future establish an action level for lead in juice at levels above 50 ppb. If you determine that lead is a hazard that is reasonably likely to occur in your juice, we recommend that you establish controls to ensure that lead levels do not exceed 50 ppb (better 30 ppb).
Consumption of juice containing excessive levels of tin can lead to acute gastrointestinal illness. The Codex Alimentarius Commission is considering establishing a maximum level of 200 ppm for tin in canned liquid foods for the protection of consumers.